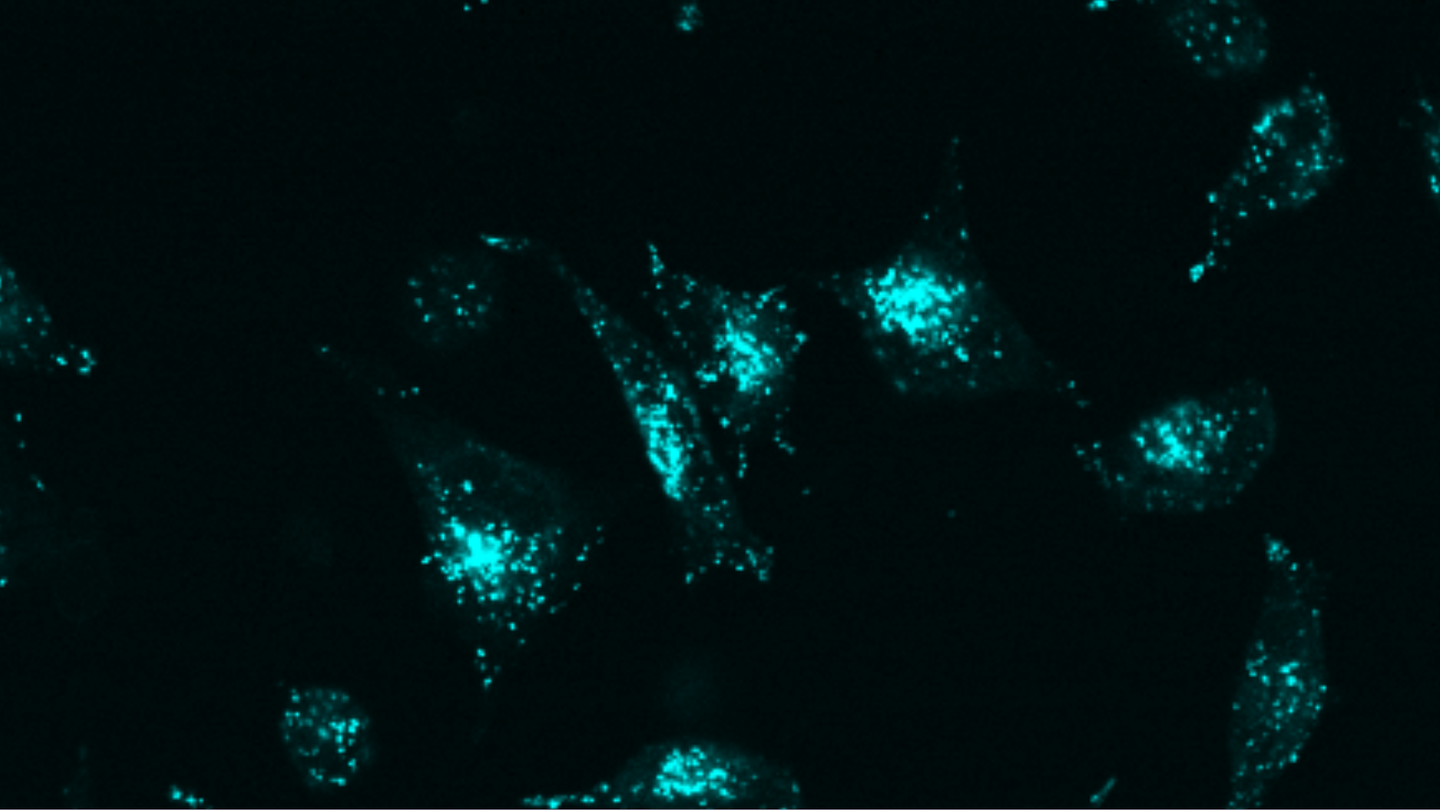
Designed endocytosis-inducing proteins degrade targets and amplify signals
Published in: Nature [Open Access]
Authors: Buwei Huang, Mohamad Abedi, Green Ahn, Brian Coventry, Isaac Sappington, Cong Tang, Rong Wang, Thomas Schlichthaerle, Jason Z. Zhang, Yujia Wang, Inna Goreshnik, Ching Wen Chiu, Adam Chazin-Gray, Sidney Chan, Stacey Gerben, Analisa Murray, Shunzhi Wang, Jason O’Neill, Li Yi, Ronald Yeh, Ayesha Misquith, Anitra Wolf, Luke M. Tomasovic, Dan I. Piraner, Maria J. Duran Gonzalez, Nathaniel R. Bennett, Preetham Venkatesh, Maggie Ahlrichs, Craig Dobbins, Wei Yang, Xinru Wang, Danny D. Sahtoe, Dionne Vafeados, Rubul Mout, Shirin Shivaei, Longxing Cao, Lauren Carter, Lance Stewart, Jamie B. Spangler, Kole T. Roybal, Per Jr Greisen, Xiaochun Li, Gonçalo J. L. Bernardes, Carolyn R. Bertozzi, David Baker
We have designed a new class of proteins that can eliminate a wide range of disease-associated molecular targets from living cells. This technology holds immense therapeutic potential, including for applications in precision cancer therapy and the management of autoimmune and neurodegenerative disorders.
This research was led by Buwei Huang, Mohamad Abedi, Green Ahn, and Brian Coventry, in close collaboration with the Bertozzi Lab at Stanford University. Extensive experimental validation was performed by collaborators at UCSF, Johns Hopkins University, UT Southwestern Medical Center, University of Cambridge, University of Lisbon, and Novo Nordisk.
This technology along with several other recent projects from the lab demonstrate that the computational design of novel proteins that bind to predetermined targets is now routinely achievable. Challenges remain for specific classes of targets and binding modes, but as we reported more than a year ago, methodological advances including our latest deep-learning methods for protein design have improved binder design success rates by a factor of ten or more.
The targeted degradation challenge
Targeted protein degradation is a promising therapeutic strategy for diseases caused by harmful proteins. Existing approaches, such as Lysosome Targeting Chimeras (LYTACs), attempt to harness the cell’s natural degradation pathways by tagging unwanted proteins for lysosomal destruction. However, these methods have limitations. They often require complex chemical modifications, making manufacturing challenging and limiting their applicability to a narrow range of targets.
“Our goal was to develop more versatile tools for degrading proteins linked to disease,” explained Green, who pioneered LYTAC technology as a graduate student in the Bertozzi Lab before joining the Baker Lab as a Jane Coffin Childs HHMI Postdoctoral Fellow. “By harnessing the power of protein design, we’ve created a new approach that uses simpler components, achieves greater precision, and can be applied to virtually any target molecule on the surface of cells.”
Introducing EndoTags
Through advanced computational methods, we created synthetic proteins called EndoTags that bind to specific cell surface receptors involved in endocytosis — the process by which cells internalize substances from their surroundings.
“We designed EndoTags to be simple to manufacture. They do not require any complex chemical modifications and, as proteins, can be genetically encoded. This should make them more accessible for therapeutic development,” said Buwei.
By attaching EndoTags to other designed proteins that recognize and bind to disease-associated targets, the team created protein-based Lysosome Targeting Chimeras, or pLYTACs capable of directing unwanted proteins to the lysosome for degradation. For example:
- Epidermal Growth Factor Receptor (EGFR): Overexpressed in many cancers, EGFR was effectively degraded using pLYTACs combining an EGFR-binding domain with our EndoTags. In cell studies, we observed a significantly greater reduction in EGFR levels compared to existing methods.
- Programmed Death-Ligand 1 (PD-L1): An immune checkpoint protein, PD-L1 was targeted using pLYTACs to enhance immune responses against tumors.
As genetically encoded protein technologies, EndoTags and pLYTACs may be deliverable via therapeutic mRNA or gene therapy methods. Moreover, because they are designed to bind to specific receptors without interfering with natural ligands, they may minimize the potential of off-target effects.
Design strategies
A key aspect of our work was the custom design of EndoTags for different receptors with distinct mechanisms and tissue distributions. Here’s how we approached this:
Constitutively cycling receptors (Sortilin and Transferrin Receptor)
These receptors naturally shuttle between the cell surface and intracellular compartments. We designed EndoTags that bind to non-overlapping sites on these receptors, ensuring they do not compete with natural ligands. By doing so, we sought to hijack these natural endocytic pathways to internalize and degrade target proteins.
- Design Process: Using Rosetta de novo protein design, we targeted epitopes on Sortilin and the Transferrin Receptor that are distant from natural ligand binding sites. This involved computationally generating thousands of candidate binders and experimentally screening them for affinity and specificity.
Receptors requiring conformational change (Insulin-like Growth Factor 2 Receptor, IGF-2R)
For receptors like IGF-2R, endocytosis is triggered by conformational changes upon ligand binding. We engineered EndoTags that bind to two distinct domains on IGF-2R, inducing the necessary structural rearrangement to trigger internalization.
- Design Process: We developed binders for two separate domains of IGF-2R and fused them to create a single molecule capable of bridging the domains. This required precise modeling of the spatial orientation and flexibility between binding domains.
Receptors triggered by custering (Asialoglycoprotein Receptor, ASGPR)
ASGPR requires receptor clustering to initiate endocytosis. We created multivalent EndoTags by linking multiple binding domains, promoting receptor clustering upon binding.
- Design Process: We designed monomeric binders to ASGPR and assembled them into dimeric or trimeric forms. This multivalency enhances avidity and effectively induces receptor clustering and subsequent internalization.
Precision targeting via logic-gates
A major advantage of computationally designed protein systems is the ability to engineer therapeutic precision at the molecular level. We demonstrated this here by combining multiple EndoTags with our previously reported LOCKR switch technology to create logic-gated pLYTACs that induce degradation only under specific conditions.
- AND Gate Mechanism: We designed pLYTACs that require the presence of two cell surface markers to initiate degradation. This enhances specificity, reducing potential side effects by targeting only cells that exhibit both markers — common in certain cancer cells but not in healthy tissue.
Next steps
We believe this collaborative work represents a significant step forward in the field of targeted protein degradation. Creating a versatile, genetically encodable technologies for protein elimination opens new possibilities for precise therapies against a wide array of diseases currently considered difficult to treat.
Looking ahead, we aim to expand the range of targets amenable to EndoTags, potentially enabling therapies for more diseases. We also plan to explore clinical applications of pLYTACs, with hopes of developing new treatments for cancer, autoimmune, and neurological disorders. Furthermore, EndoTags may help scientists better understand fundamental aspects of cell biology, contributing to new insights into the complex mechanisms of disease.
This work underscores the transformative potential of modern protein design, where new biological functions can be created rather than discovered.
Support
We thank all our collaborators who contributed to this project. Special thanks to Dr. Carolyn Bertozzi and her team for their invaluable insights and collaboration. This research was funded by several organizations, including the National Science Foundation, National Institutes of Health, Department of Defense, Howard Hughes Medical Institute, Open Philanthropy, and The Audacious Project. All funders are listed in the manuscript.