In a new study in Science, we introduce an AI-assisted drug discovery approach and use it to generate over 14.9 million never-before-seen peptides with appealing pharmaceutical features, including molecules that inhibit key proteins linked to coronavirus infection and cancer in the lab.
This research was led by Patrick Salveson, a recent postdoc in the lab. It led to a spinout company, Vilya, which emerged from the Institute for Protein Design in 2022. David Baker, Patrick, two other recent lab members, and UW medicinal chemistry professor Gaurav Bhardwaj are among Vilya’s co-founders.
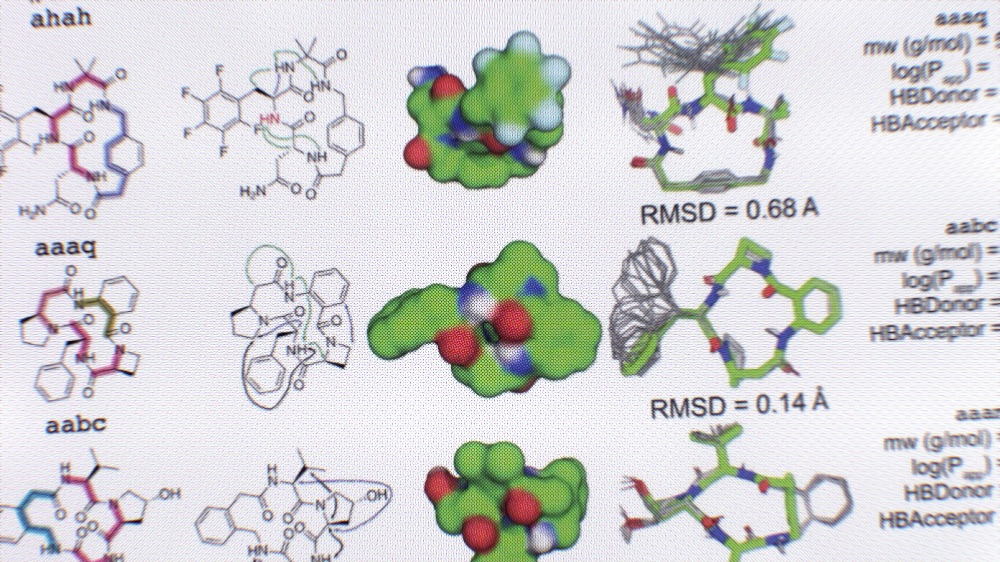
Modeling Millions of Macrocycles
The molecules developed in the study, which are ring-shaped peptides called macrocycles, have the potential to treat a wide range of health disorders. In nature, this class of compounds is known to shut down pain signals, viruses, and more. Semaglutide (brand name: Ozempic, Wegovy) is an example of a peptide medicine.
“Certain snails, sponges, and other marine animals produce macrocycles with potent activities, and scientists have had some success turning these natural products into medicines. But until now, there hasn’t been a way to systematically create new macrocycles that might treat specific diseases. Our work shows that this promising class of chemicals can be systematically explored using computational design.”
— Senior author Patrick Salveson, PhD
By creating software that can design millions of never-before-seen macrocycle arrangements, this research may unlock peptides with appealing pharmaceutical features, such as the ability to cross cell membranes to reach disease targets deep inside cells.
The number and diversity of macrocycles described in this study far exceed what has been found in nature, and roughly half satisfy the so-called ‘rule-of-five’ criteria for drug-like compounds.
“The challenge here was to come up with an efficient way to model these chemicals on the computer. We found a solution that combines the accuracy of AIMNet, which is an AI-powered tool for simulating quantum mechanics, with the speed of a more traditional software approach. We optimized these steps to create a new way to quickly build ring-shaped compounds.”
— Study author Adam Moyer, PhD
AIMNet was developed in 2019 by scientists from Los Alamos National Laboratory, Jackson State University, and the University of North Carolina at Chapel Hill.
Validating Structure and Function in the Lab
To demonstrate the reliability of this new design approach, the team manufactured and tested a small number of macrocycles. Researchers from the Core R&D Labs at the Institute for Protein Design contributed to this work.
Almost all synthesized macrocycles resisted degradation by serum proteases for more than 24 hours and readily crossed artificial cell membranes, suggesting these peptides have the potential to traverse through the body and enter cells.
As intended, some macrocycles also specifically inhibited HDAC6, a protein linked to cancer, without affecting other similar proteins. This could lead to cancer treatments with fewer side effects.
Additionally, from millions of macrocycles, we pinpointed one that could inhibit an essential protein from the virus that causes COVID-19. Further optimization enhanced effectiveness, demonstrating the method’s potential to generate new antivirals.
Funding
This study received support from several sources. All funders are listed in the manuscript.
Expansive discovery of chemically diverse structured macrocyclic oligoamides
Authors: Patrick J. Salveson, Adam P. Moyer, Meerit Y. Said, Gizem Gӧkçe, Xinting Li, Alex Kang, Hannah Nguyen, Asim K. Bera, Paul M. Levine, Gaurav Bhardwaj, David Baker.