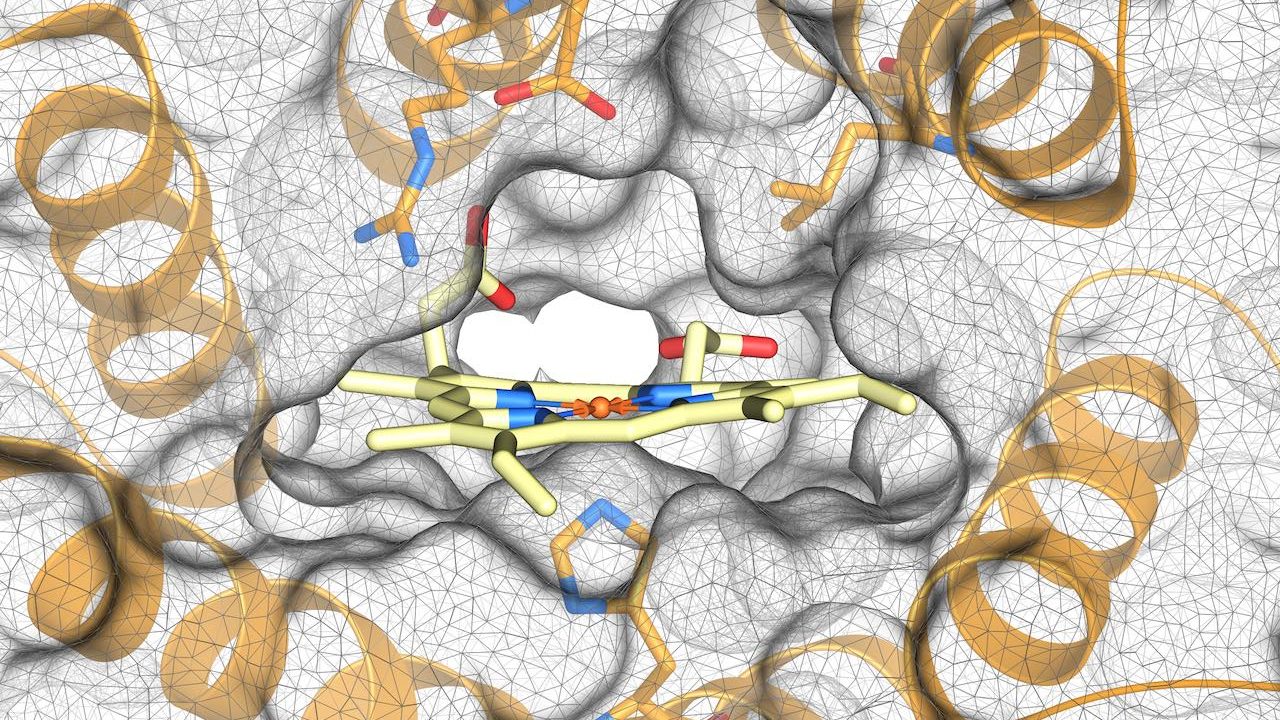
Today we report in JACS [Open Access] the design of enzymes that contain heme, an important iron-containing molecule involved in many biological processes. The design was inspired by the structure and function of natural heme-binding enzymes, including cytochrome P450, which are involved in a wide range of chemical reactions both inside the body and beyond.
Performed in collaboration with the Green Lab at the University of Manchester, this project was led by Baker Lab postdocs Indrek Kalvet, PhD and Anindya Roy, PhD, and Green Lab postdocs Mary Ortmayer, PhD, and Jingming Zhao, PhD.
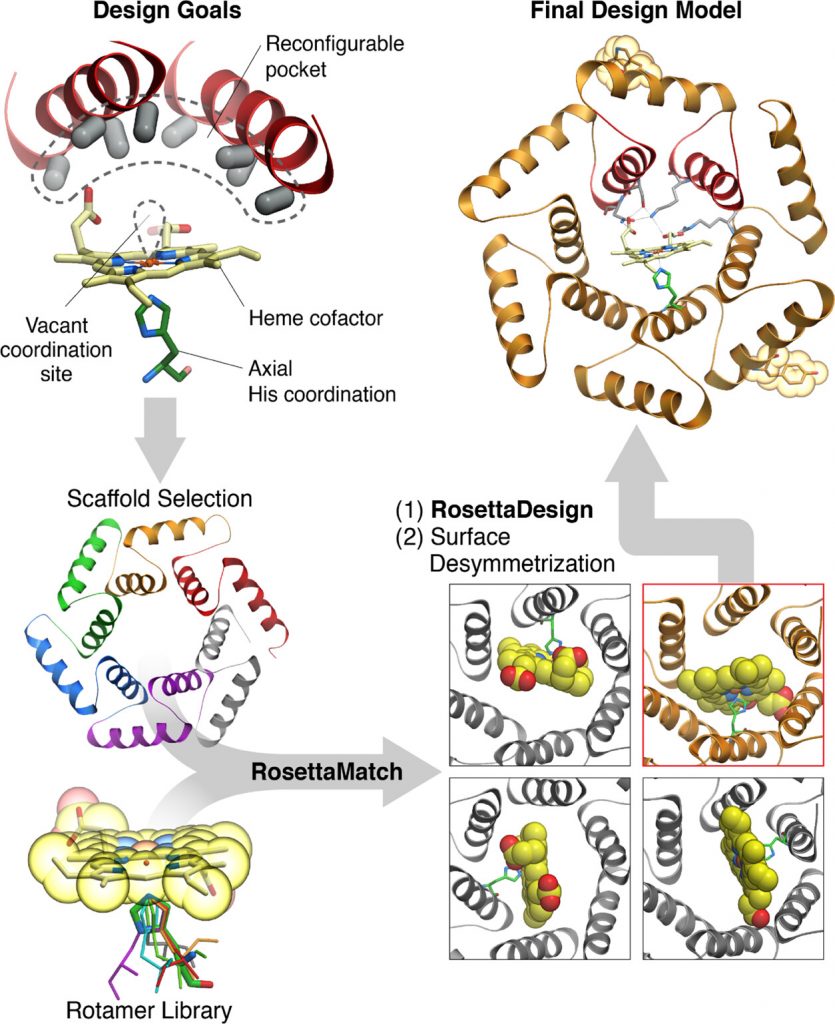
Figure 1. Schematic of the approach to computational design. To generate heme-binding proteins containing a reconfigurable pocket near a free coordination site on the Fe atom (top left), a Heme-HIS moiety was matched into the pore of helical solenoid scaffolds of appropriate size (bottom left and right). The sequence around the heme cofactor was then optimized using Rosetta (top right).
The team used computational methods to design dnHEM1, a helical solenoid protein that has a large central cavity that can bind to a variety of substrates and a highly stable structure. The heme binding site was generated using Rosetta Match and Design. The protein was produced in E. coli and its structure was confirmed using X-ray crystallography. We found that dnHEM1 binds to heme very tightly, with a dissociation constant of less than 10 nanomolar.


We then used directed evolution, a method that mimics natural evolution, to improve the peroxidase activity of dnHEM1. Peroxidases are enzymes that catalyze the oxidation of various substrates by hydrogen peroxide. The most active variant, dnHEM1.2, contains three mutations and is able to catalyze the oxidation of a model substrate with a rate constant of 129.5 per second.
Finally, the team turned to computational methods to redesign dnHEM1 to catalyze the cyclopropanation of olefins, a reaction that creates two new stereocenters and is useful for the synthesis of bioactive molecules. Rather than relying on directed evolution, we were able to create variants of dnHEM1 that catalyze the reaction with high enantioselectivity, meaning they preferentially produce one enantiomer (a molecule that is a mirror image of another molecule) over the other.
This study demonstrates the potential of protein design to create enzymes with cofactors tuned for specific activities. This approach could be used to design enzymes that catalyze a wide range of chemical reactions, extending the catalytic potential of heme beyond natural scaffolds, as well as to other transition metals.