Koga, N., Tasumi-Koga R., et al., Nature. 491(7423), 222-227. (2012)
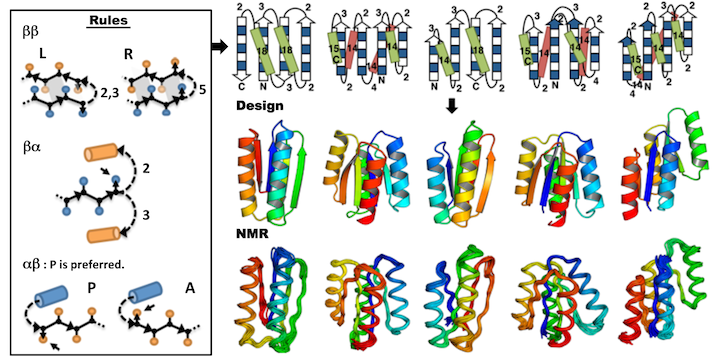
We describe an approach to designing ideal protein structures stabilized by completely consistent local and non-local interactions. The approach is based on a set of rules relating secondary structure patterns to protein tertiary motifs, which make possible the design of strongly funneled protein folding energy landscapes. Guided by these rules, we designed sequences predicted to fold into ideal protein structures consisting of alpha helices, beta strands, and minimal loops. Designs for five different topologies were found to be monomeric, very stable, and adopt structures in solution nearly identical to the computational models. These results illuminate how the folding funnels of natural proteins arise and provide the foundation for engineering a new world of functional proteins free from natural.