De Novo Design of Integrin α5β1 Modulating Proteins to Enhance Biomaterial Properties
Published in: Advanced Materials
Authors: Xinru Wang, Jordi Guillem-Marti, Saurav Kumar, David S. Lee, Daniel Cabrerizo-Aguado, Rachel Werther, Kevin Alexander Estrada Alamo, Yan Ting Zhao, Adam Nguyen, Irina Kopyeva, Buwei Huang, Jing Li, Yuxin Hao, Xinting Li, Aritza Brizuela-Velasco, Analisa Murray, Stacey Gerben, Anindya Roy, Cole A. DeForest, Timothy Springer, Hannele Ruohola-Baker, Jonathan A. Cooper, Melody G. Campbell, Jose Maria Manero, Maria-Pau Ginebra, David Baker


In a new study, we introduce a completely new class of proteins called NeoNectins that help the body respond to surgical implants, healing gels, and other biomaterials. This project was led by postdoctoral scholar Xinru Wang, PhD, and visiting researcher Jordi Guillem-Marti, PhD.
When cells and materials meet
Modern implants and wound dressings often contain special coatings meant to promote healing. These coatings, however, often rely on chemical signals that cells don’t fully recognize or respond to properly. New tools that allow biomaterials to better connect with specific cells could improve health outcomes following surgery, injury, or even normal aging.
Our bodies are made up of trillions of cells. Most anchor themselves in place by grabbing onto nearby tissues. One key molecule in this process is a receptor called integrin α5β1, which helps cells stick to the extracellular matrix, the supportive structure around the cells. It also helps cells sense their environment and respond to local signals that guide them to grow, divide, and migrate.
Introducing NeoNectins
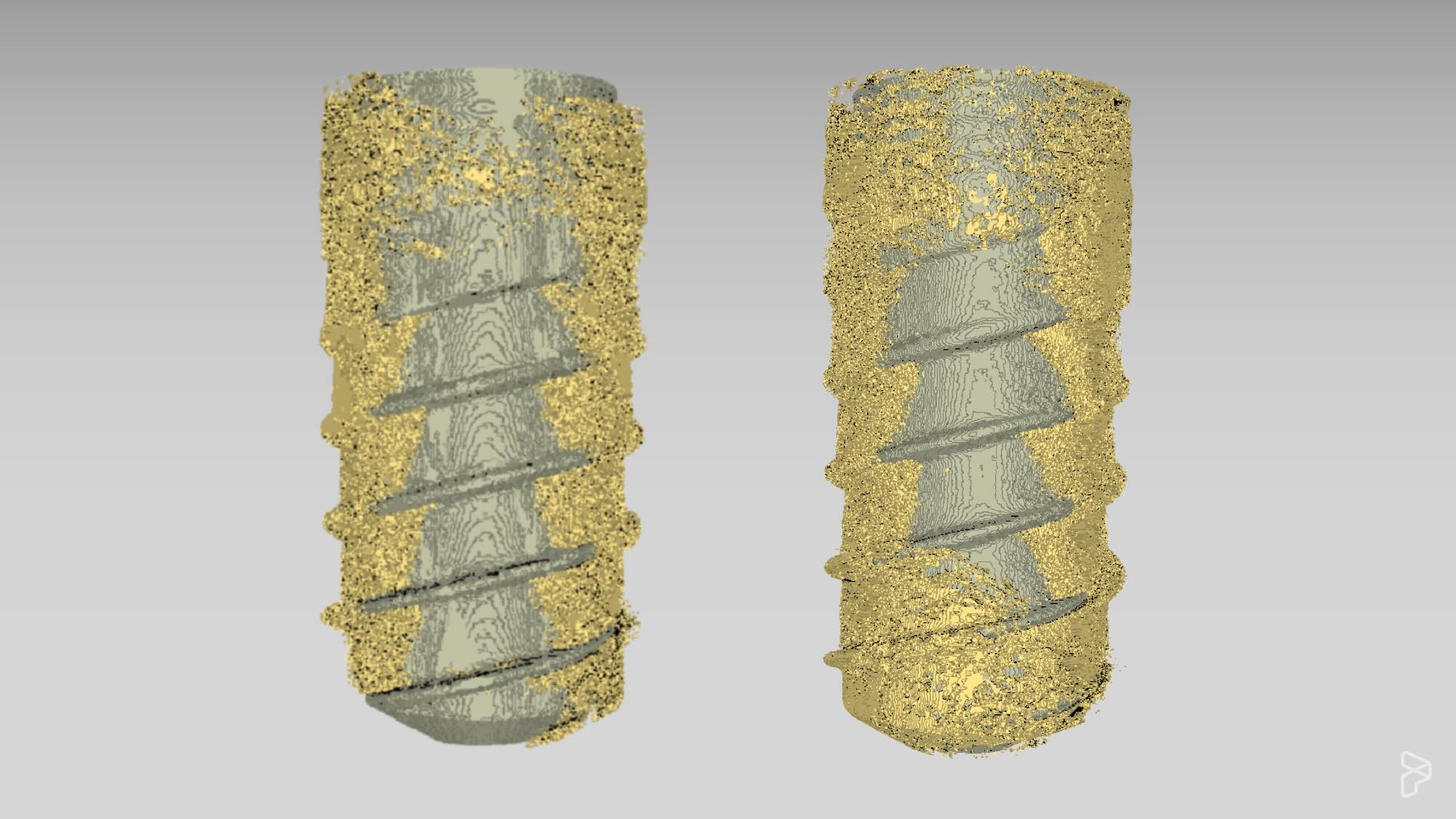
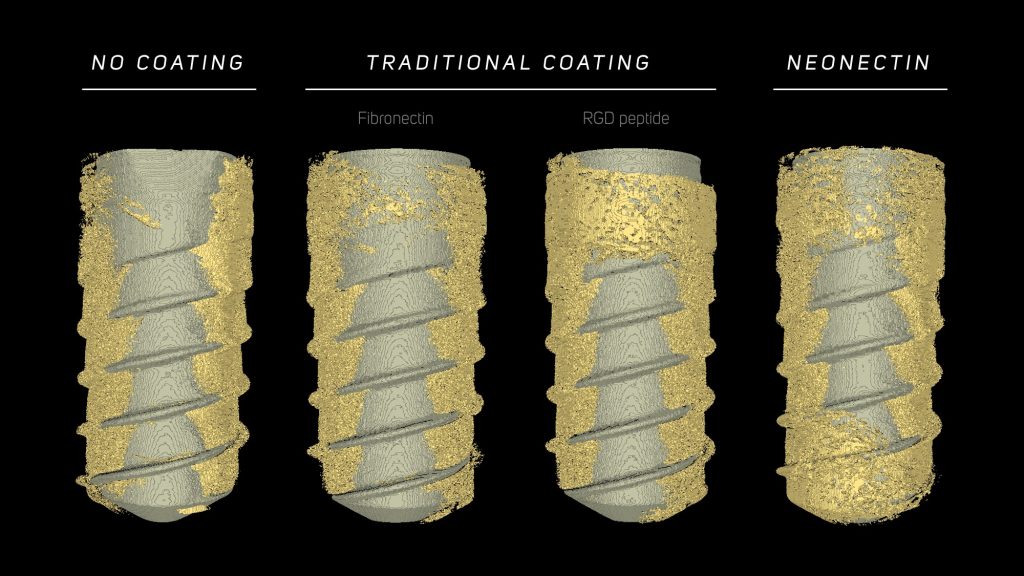
NeoNectins are tiny proteins designed to bind and activate integrin α5β1, telling cells — especially those important for regeneration — it’s safe to stay and start building new tissue where it is needed. When grafted onto titanium implants or embedded into hydrogels, NeoNectin-enhanced materials performed better than other leading coatings, supporting stronger cell adhesion, more robust gene expression, and even greater bone formation around bone implants in animals.
Smarter biomaterials
Built from scratch using protein design software, these small proteins stabilize integrin α5β1 in its active form. And unlike older biomaterial coatings that rely on chemical signals like RGD peptide or natural proteins like fibronectin, NeoNectins were custom-built to provide a clear, targeted message to the body.
“Our goal was to design proteins that actively engage the body’s natural healing pathways,” said Xinru. “NeoNectins give us a new way to build functional biomaterials — and that opens the door to smarter, more effective treatments.”
This project was led by the Institute for Protein Design at UW Medicine and included collaborators from the University of Washington, Fred Hutchinson Cancer Center, Harvard University, and Spain’s Universitat Politecnica de Catalunya – BarcelonaTech. All funders are listed in the manuscript.