Publication
Bottom-up design of Ca2+ channels from defined selectivity filter geometry
Authors: Yulai Liu, Connor Weidle, Ljubica Mihaljević, Joseph L. Watson, Zhe Li, Le Tracy Yu, Sagardip Majumder, Andrew J. Borst, Kenneth D. Carr, Ryan D. Kibler, Tamer M. Gamal El-Din, William A. Catterall, David Baker
Lead Authors

Coverage
Designing Ca2+ Channels from Filter Geometry — bioengineering.org
Signal transduction — the process by which cells sense and respond to their environment — is essential for life. Among the key players in this process are calcium ions, which drive electrical activity in excitable cells and regulate diverse signaling events such as muscle contraction, heartbeat, and neurotransmitter release. Specialized proteins known as calcium channels allow cells to control calcium influx, even when higher concentrations of sodium ions are around.
This week we report the design of new calcium channels built entirely from scratch. Led by postdoctoral scholar Yulai Liu, PhD, this project demonstrates that even complex biochemical functions that remain only partially understood can now be built from first principles using artificial intelligence.
A fundamental challenge
Natural calcium channels have been adapted into research tools, but these modified molecules are delicate and thus challenging to work with. Creating ion channels that are simpler and that achieve precise ion selectivity has remained a major challenge.
“Biochemists have been studying ion channels for decades and debating about how they work for almost as long. We set out to build new versions to allow biologists to precisely control cellular signaling.”
David Baker, PhD
Building from the inside out
The team used RFdiffusion to build calcium channels by starting with the selectivity filter, the critical structure that allows calcium ions through while blocking similar ions like sodium. They then generated supporting protein structures outward from this feature.
Unlike water-soluble proteins, which make up most known structures in protein databases, ion channels function within lipid membranes and adopt different structural folds and functional mechanisms. The team had to adapt their design tools to generate pore-containing transmembrane protein structures and amino acid sequences that would reliably fold as transmembrane ion channel proteins.
“By engineering channels that can be precisely controlled, we hope to study, and eventually manipulate, cellular behaviors in entirely new ways.”
Yulai Liu, PhD
Validating the new channels
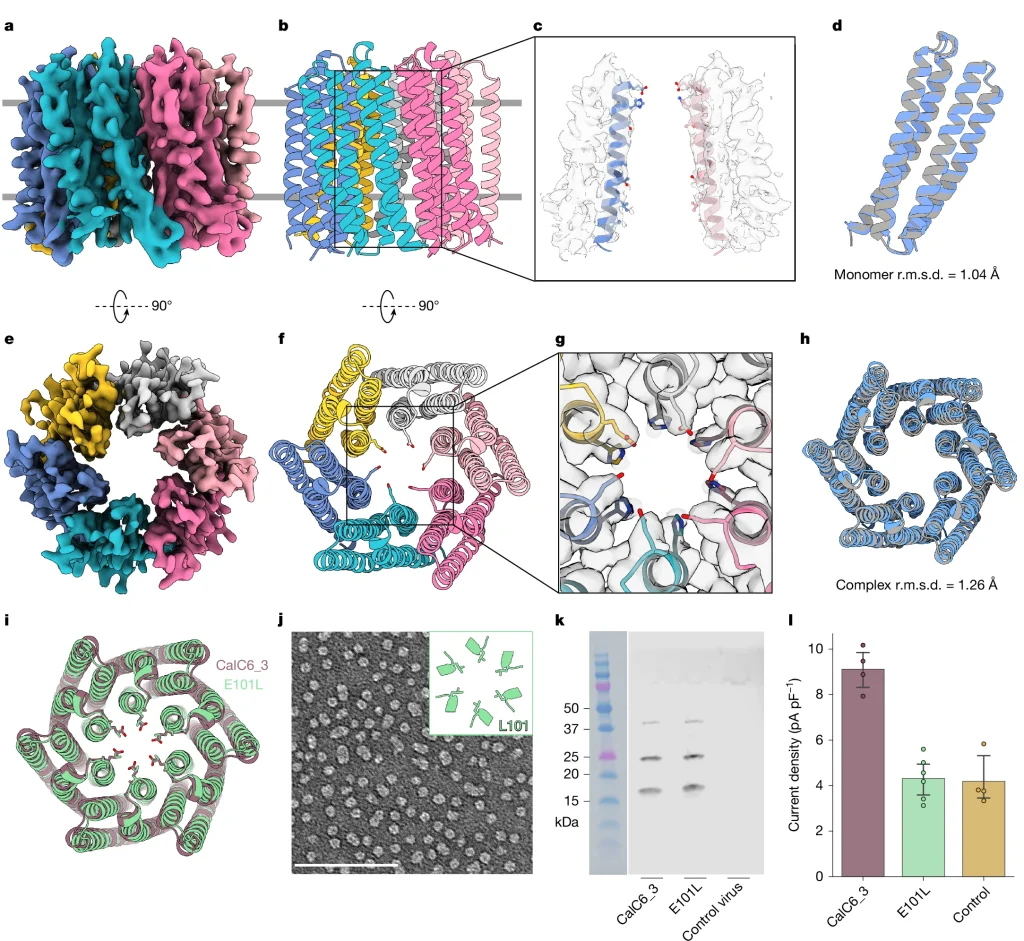
The designed channels were produced in insect cells and studied using patch-clamp electrophysiology, the gold standard for evaluating ion channel function. Multiple designs conducted calcium as intended and achieved calcium selectivity, transmitting about five times more current for calcium compared to sodium ions.
Cryo-electron microscopy revealed that one functional channel assembled exactly as designed — the protein backbone matched the computational model with atomic precision. The IPD EM Research Core carried out the cryo-EM characterizations and structural determination of the designed channel.
Implications for neuroscience and beyond
The team envisions applying this design pipeline to explore the general physical principles underlying ion selectivity, especially for creating channels for metal ions beyond those found in nature. These efforts could deepen understanding of how ions move through proteins embedded in membranes and how such mechanisms support complex processes like brain signaling and immune cell activation.
Synthetic ion channels could serve as new tools for research, from neuroscience experiments to cardiac biology models and synthetic signaling circuits.
Remembering Bill Catterall
UW professor of pharmacology William Catterall, PhD, a luminary figure in the field of ion channel research, contributed to this study and sadly passed away in 2024 at age 77. Over his more than 50-year research career, Bill was the first to isolate sodium and calcium channels in the 1980s and made fundamental contributions to understanding their structure, function, and role in disease.
The Catterall Lab in the UW Department of Pharmacology provided essential expertise in electrophysiology that guided both structural and functional characterization of the designed proteins. Yulai was co-mentored by Professor Catterall, whose mentorship was pivotal to the project’s success.
Funding
This research was funded by The Audacious Project, Howard Hughes Medical Institute, Gates Foundation (INV-043758), Wu Tsai Protein Innovation Fund, Open Philanthropy, Alexandria Venture Investments Translational Investigator Fund, Nan Fung Life Sciences Translational Investigator Fund, BASF Corporation, and United States Air Force Office of Scientific Research (FA9550-22-1-0506), Department of Energy (DE-SC0018940), and National Institutes of Health (R01HL112808, R01AG063845).