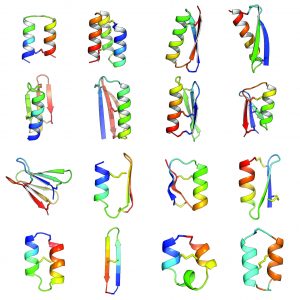
Small constrained peptides combine the stability of small molecule drugs with the selectivity and potency of antibody-based therapeutics. However, peptide-based therapeutics have largely remained underexplored due to the limited diversity of naturally occurring peptide scaffolds, and a lack of methods to design them rationally.
In an article published in Nature this week, Baker lab scientists and collaborators describe the development of computational methods for de novo design of constrained peptides with exceptional stabilities. They used these computational methods to design 18-47 residue constrained peptides with diverse shapes and sizes. The designed peptides presented in the paper cover three broad categories: 1) genetically encodable disulfide cross-linked peptides, 2) synthetic disulfide cross-linked peptides with non-canonical sequences, and 3) cyclic peptides with non-canonical backbones and sequences. Experimentally determined structures for these peptides are nearly identical to their design models.
By including D-amino acids (mirror images of the L-amino acids), and thus expanding the palette of building blocks, Baker lab scientists designed peptides in a sequence and structure space sampled rarely by Nature. Indeed, the article describes successful design of a cyclic 2-helix peptide of mix chirality that represents a shape beyond natural secondary- and tertiary structure.
These designed peptides also exhibit exceptional stability to thermal and chemical denaturation, and thus could serve as attractive scaffolds for design of novel peptide-based therapeutics. More broadly, development of this new computational toolkit to precisely design constrained peptides opens the door for “on-demand” development of a new generation of peptide-based therapeutics.